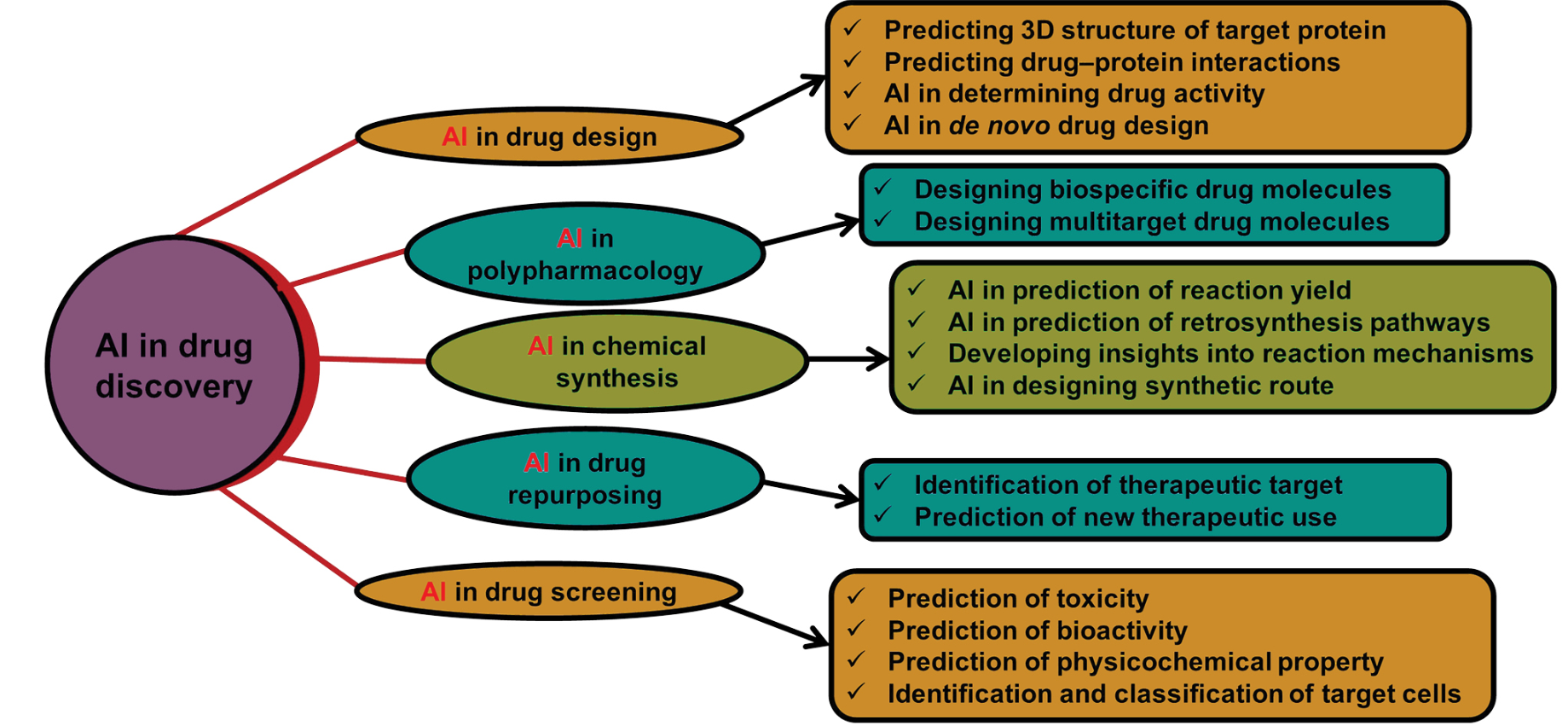
AI can make drug-testing precise, and relevant to human biology
(Source – The Hindu, International Edition – Page No. – 7)
|
Topic: GS3 – Science and Technology |
|
Context |
|
Rising Use of AI in Drug Approval and Drug Testing
-
The U.S. FDA has noted a sharp increase in the use of AI in drug development submissions.
-
In 2016 and 2017, there was only one AI-related submission per year, but this tripled in the next two years.
-
By 2021, there were 132 AI-related submissions, showing a tenfold increase from the previous year.
Challenges in Conventional Drug Development
-
Traditional drug development takes nearly 10 years and costs over a billion dollars.
-
The success rate of conventional (animal-based) drug testing is only 14%.
-
Animal testing does not always accurately predict human responses due to differences in metabolism and genetic variability.
|
Role of AI in Drug Development |
|
AI in Predicting Drug Safety
-
AI-based models can assess a drug’s potential risks before human trials begin.
-
AI can predict how a chemical compound might affect different organs, helping reduce unexpected adverse effects.
-
A recent research study introduced a “safety toolbox” that integrates multiple data types, such as chemical properties and exposure levels, to predict toxicity risks.
Challenges of Using AI in Drug Testing
-
The accuracy of AI models depends on the quality of the data they are trained with.
-
If AI is trained on biased or incomplete data, its predictions may not be reliable.
-
Transparency is another issue, as many AI models do not disclose their internal workings or training data.
FDA’s Draft Guidelines on AI in Drug Development
-
The FDA has proposed a stepwise framework to assess the credibility of AI models.
-
It emphasizes:
-
Identifying specific research questions AI will address.
-
Evaluating AI model risks, especially for incorrect predictions with life-threatening consequences.
-
Improving data quality and reducing biases to enhance AI model reliability.
-
Continuous monitoring and maintenance of AI models throughout their lifecycle.
-
-
The guidelines particularly focus on AI’s role in preclinical drug testing to assess safety before human trials.
Global and Indian Efforts in AI Regulation
-
Other regulatory bodies have also released AI-related drug development guidelines.
-
In 2023, India’s New Drugs and Clinical Trials (Amendment) Rules allowed AI-generated data to assess drug safety, reducing reliance on animal trials.
Impact of AI Guidelines
-
The guidelines help align government policies, industry expectations, research strategies, and consumer safety.
-
They act as a stable reference point for stakeholders to ensure AI is used effectively in drug development.
|
PYQ: Introduce the concept of Artificial Intelligence (AI). How does AI help clinical diagnosis? Do you perceive any threat to privacy of the individual in the use of Al in healthcare? (150 words/10m) (UPSC CSE (M) GS-3 2023) |
|
Practice Question: How is Artificial Intelligence (AI) transforming drug development and safety assessment? What challenges does it pose, and how do recent regulatory guidelines address them? (250 Words /15 marks) |