Government Bans 156 Fixed-Dose Combination Drugs, Targeting Irrational and Ineffective Medications
(Source: Indian Express; Section: Explained;)
| Topic: GS2 – Social Justice – Health
GS3 – Science and Technology |
| Context: |
|
Analysis of News:
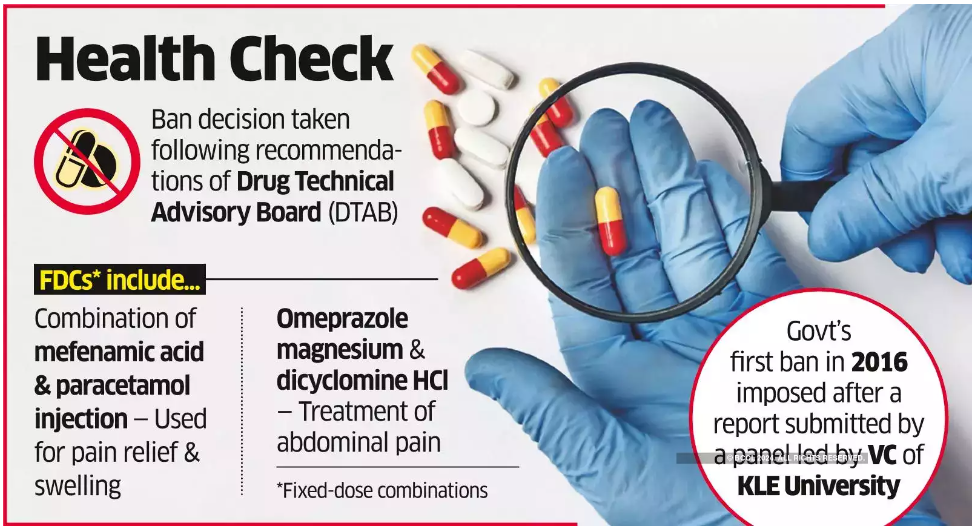
Understanding Fixed-Dose Combinations (FDCs)
- Definition: FDCs are medications that contain more than one active ingredient in a single dosage form, such as a pill, capsule, or injection.
- Purpose: These combinations are designed to help patients, especially those with chronic conditions like tuberculosis and diabetes, by reducing the number of pills they need to take daily, thereby improving treatment adherence.
- Issue: The use of FDCs can sometimes result in patients taking drugs they don’t need, leading to unnecessary consumption of medications.
Specific FDCs Banned
The banned FDCs include:
- Gastrointestinal Treatments: Various enzyme combinations.
- Anti-Allergic Combinations: Including levocetirizine with nasal decongestants, and syrups for mucus breakdown.
- Skin Condition Treatments: Combinations involving menthol, aloe vera, vitamin E, and antiseptics.
- Migraine Treatments: FDCs combining migraine relief with anti-nausea drugs.
- Menstrual Cramp Relief: Mefenamic acid combined with tranexamic acid.
- Erectile Dysfunction: Sildenafil combined with muscle relaxants.
Availability and Immediate Impact
- Market Availability: Manufacturers are required to stop production, stocking, and sale of these drugs immediately. However, these medications may still be available in the market due to legal challenges allowing the sale of existing stock.
- Health Impact: For consumers, taking one of these banned FDCs now is unlikely to cause harm, but the ban was necessary to prevent irrational drug use.
Reasons Behind the Ban
- Irrational Combinations: The banned FDCs often contain ingredients that either do not work well together or are unnecessary when combined.
- Antibiotic Resistance: A significant concern is the overuse of antibiotics, which can lead to increased antibiotic resistance. Banning irrational combinations helps reduce unnecessary antibiotic consumption.
- Price Control Evasion: Some companies create FDCs to avoid government-imposed price controls on essential medicines.
Timing and Context of the Ban
- Regulatory Reforms: The ban aligns with the government’s efforts to eliminate irrational drug combinations that were previously approved by state authorities without proper trials. The 2019 regulations classify FDCs as new drugs, requiring central approval.
- Historical Background: The issue of irrational drug combinations was first highlighted by a parliamentary panel in 2012. A 2014 committee reviewed 3,450 FDCs and identified 963 as irrational, leading to the progressive banning of such drugs.
| What is the Central Drugs Standard Control Organisation (CDSCO)? |
|
| Practice Question: Discuss the rationale behind the Indian government’s recent ban on 156 Fixed-Dose Combination (FDC) drugs. What are the potential impacts of this ban on public health and the pharmaceutical industry? (250 words/15 m) |
