9 November 2024 : PIB Summary For UPSC
1. INDIA- AUSTRALIA JOINT MILITARY EXERCISE AUSTRAHIND COMMENCES IN MAHARASHTRA
(Source – https://pib.gov.in/PressReleseDetail.aspx?PRID=2071767®=3&lang=1 )
| Context |
| The 3rd edition of Exercise AUSTRAHIND commenced at the Foreign Training Node in Pune, Maharashtra, on November 8, 2024, and will conclude on November 21, 2024.This annual event alternates between India and Australia, with the previous edition held in Australia in December 2023. |
3rd Edition of Joint Military Exercise AUSTRAHIND:
- Location: Foreign Training Node, Pune, Maharashtra
- Duration: 8th to 21st November 2024
- Participants:
- India: 140 personnel, primarily from DOGRA Regiment and 14 from the Indian Air Force
- Australia: 120 personnel from the 13th Light Horse Regiment, 10th Brigade of the 2nd Division
- Aim: Enhance military cooperation and interoperability between India and Australia for joint sub-conventional operations in semi-urban and semi-desert terrains under UN Chapter VII.
- Objective: Share best practices, develop camaraderie, and strengthen tactical skills
- Key Drills/Operations:
- Terrorist action response
- Joint counter-terrorism operations
- Raid, Search and Destroy Missions
- Securing a helipad, drone operations, Special Heli Borne Operations
2. Union Minister for Chemicals and Fertilizers Shri JP Nadda launches ” Scheme for Strengthening the Medical Device Industry “
(Source – https://pib.gov.in/PressReleseDetail.aspx?PRID=2071850®=3&lang=1 )
| Context |
| The Union Minister of Chemicals & Fertilisers and Health & Family Welfare launched a new comprehensive scheme to boost the medical device industry with a total outlay of 500 crores.The scheme targets key areas including manufacturing of components, skill development, clinical studies, infrastructure development, and industry promotion. |
Objective of the Scheme
- The initiative aims to enhance India’s self-reliance in medical devices, promote competitiveness, and ensure the growth of the industry.
- The Minister emphasised that this scheme would act as a game changer, contributing to India’s vision of becoming self-reliant.
- The government assures full support to the industry in utilising this scheme effectively.
Importance of Medical Devices in Healthcare
- Medical devices are essential for prevention, diagnosis, and treatment in healthcare, including diagnostic machines, surgical instruments, stents, and prosthetics.
- India’s medical device market is valued at approximately $14 billion and is projected to reach $30 billion by 2030.
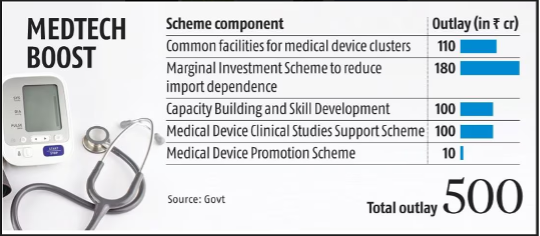
Sub-Schemes Under the Initiative
- Common Facilities for Medical Devices Clusters (Outlay: Rs. 110 Crore)
- Financial assistance for establishing common infrastructure such as R&D labs, design centres, and testing facilities in medical device clusters.
- Marginal Investment Scheme for Reducing Import Dependence (Outlay: Rs. 180 Crore)
- Aims to reduce reliance on imports by supporting manufacturing of components and raw materials domestically. Offers a one-time capital subsidy of 10-20%, capped at Rs. 10 crore per project.
- Capacity Building and Skill Development (Outlay: Rs. 100 Crore)
- Supports training and courses for developing a skilled workforce for designing and developing medical technologies. Provides financial aid for Masters and short-term courses.
- Medical Device Clinical Studies Support Scheme (Outlay: Rs. 100 Crore)
- Provides financial support for clinical studies, including animal studies and human trials, to validate MedTech products.
- Medical Device Promotion Scheme (Outlay: Rs. 10 Crore)
- Supports industry associations and export councils for organising promotional activities, conferences, and surveys.
Future Outlook
- The government’s support and strategic schemes aim to position India as a leader in the global medical device market.
- The initiatives are expected to address long-standing challenges in the sector and foster innovation and self-reliance in medical device manufacturing.
| Practice Question: India’s medical device industry is poised for significant growth, with the government introducing a comprehensive scheme to enhance self-reliance and reduce import dependency. Discuss the key components of this scheme and evaluate its potential impact on the medical device manufacturing sector in India. (250 Words /15 marks) |


