Biochemical Cycles
For last 1 billion years, atmosphere and hydrosphere have been composed of same balance of chemical components. The balance of chemical elements is maintained by a cyclic passage through the tissues of plants and animals.
Living organisms exist and survive in a diversity of associations – survival involves the presence of systemic flows such as flows of energy, water and nutrients. These flows show variations in different parts of the world, in different seasons of the year and under varying local circumstances.
Sun is basic source of energy, which initiates life processes in biosphere through photosynthesis. Half of this energy is used by plants for respiration and remaining part temporarily stored or shifted to other portions of plants. Photosynthetic organisms are consumed by the consumers in order to avail this energy.
| 16 Essential Nutrients for Plants |
| Air supplies: C [Macro]
Water supplies: H [Macro] Both: O comes from air, but also from water (through Photosynthesis) [Macro] Soil supplies:
|
Nutrient Cycle:
Nutrient Cycle (or biochemical cycle): movement of nutrient elements through the various components of an ecosystem. Cyclic movements of chemical elements of the biosphere between the organism and the environment are referred to as biogeochemical cycles.
Biochemical cycles can be classified into two categories:
- Gaseous: Reservoir of cycle exists in atmosphere; For example, CO2, N2.
- Sedimentary: Reservoir is located in earth’s crust; Phosphorus cycle, Sulphur cycle.
Factors affecting the cyclic movement of Nutrients
- Standing rate: The amount of nutrients, such as carbon, nitrogen, phosphorus, calcium, etc., present in the soil at any given time.
- Reservoir: used to meet the deficit which occurs due to imbalance in the rate of influx or efflux.
- Environmental factors: soil moisture, pH, Temp etc. regulate the rate of release of nutrient into atmosphere.
Hydrological Cycle:
Water is a cyclic resource. Hydrological cycle is the continuous movement of water within the Earth and atmosphere. It is a complex system that includes many different processes.
- Liquid water evaporates into water vapour, condenses to form clouds, and precipitates back to earth in the form of rain and snow.
- Water in different phases moves through the atmosphere (transportation). Liquid water flows across land (runoff), into the ground (infiltration and percolation), and through the ground (groundwater).
- Groundwater moves into plants (plant uptake) and evaporates from plants into the atmosphere (transpiration). Solid ice and snow can turn directly into gas (sublimation). The opposite can also take place when water vapour becomes solid (deposition).
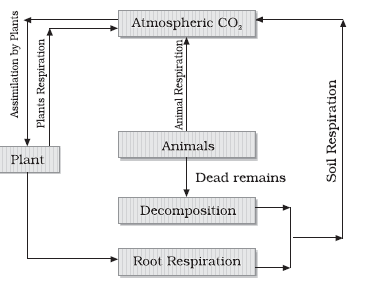
Carbon cycle:
Help of half a million carbon compounds. Carbon constitutes 49% of dry weight of organisms and is next only to water. If we look at the total quantity of global carbon, we find that 71% carbon is found dissolved in oceans. Fossil fuel also represent a reservoir of carbon. Carbon cycling occurs through atmosphere, ocean and through living and dead organisms.
- Producers: Initiated with Carbon fixation: Photosynthesis: Carbohydrate, glucose, starch, cellulose etc.
- Consumers: Plant tissue eaten by animals or get decomposed.
- Respiration.
- Herbivores convert some carbohydrate to CO2 remaining decomposed after animal dies.
- A considerable amount of carbon returns to the atmosphere through respiratory activities of the producers and consumers.
- Decomposers also contribute substantially to CO2 pool by their processing of waste materials and dead organic matter of land or oceans.
- Carbohydrates decomposed by microbes gets oxidised into CO2
- Burning of wood, forest fire and combustion of organic matter, fossil fuel, volcanic activity are additional sources for releasing CO2 in the atmosphere.
- Atmospheric inputs of Carbon through rainfall are significant. Some amount of the fixed carbon is lost to sediments and removed from circulation.

Oxygen Cycle:
- Cycling of Oxygen is highly complex process.
- It is main by-product of photosynthesis.
- It is involved in the oxidation of carbohydrates with release of energy, CO2 & water.
- It occurs in number of chemical forms – nitrates, oxides (Fe, Al), Much of Oxygen is produced from decomposition of water molecules by sunlight. Released during Photo synthesis.
Nitrogen Cycle:
Essential constituent of different organic compounds such as the amino acids, nucleic acids, proteins, vitamins and pigments.
- Nitrogen Fixing:Only a few types of organisms like certain species of soil bacteria and blue green algae are capable of utilising it directly in its gaseous form. Generally, nitrogen is usable only after it is fixed which is generally in the form of Ammonia (Ammonification).
- 90% of fixed nitrogen is biological. The principal source of free nitrogen is the action of soil micro-organisms and associated plant roots on atmospheric nitrogen found in pore spaces of the soil.
- Nitrogen can also be fixed in the atmosphere by lightning and cosmic radiation.
- In oceans, some marine animals can fix it.
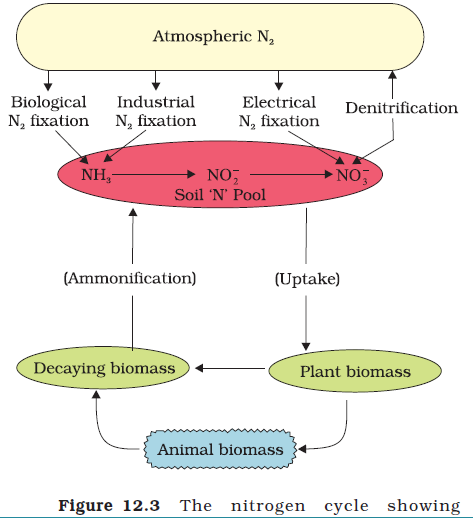
- Nitrification: Dead plants and animals, excretion of nitrogenous wastes is converted into nitrites by the action of bacteria present in the soil [Uses Oxygen from air]. Some bacteria can even convert nitrites into nitrates that can be used again by green plants.
- Conversion: Green plants can assimilate (uptake) it, and convert it to amino acids.
- Consumption: Herbivorous animals feeding on plants, consume some of it.
- There are still other types of bacteria capable of converting nitrates into free nitrogen, a process known as Denitrification.
- Using Fertilizer – Introduces ammonia directly.,

Phosphorus Cycle:
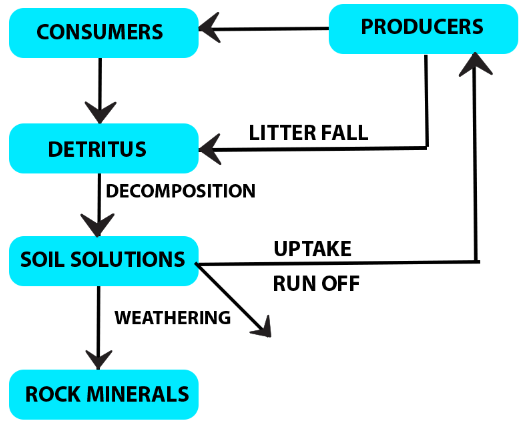
- Major constituent of biological membranes, nucleic acids and cellular energy transfer systems. Many animals also need large quantities of this element to make shells, bones and teeth.
- The natural reservoir of phosphorus is rock (phosphates),
- When rocks are weathered, minute amounts of these phosphates dissolve in soil solution and are absorbed by the roots of the plants
- Herbivores and other animals obtain this element from plants.
- The waste products and the dead organisms are decomposed by phosphate–solubilising bacteria releasing phosphorus.
Other mineral cycles:
- Mineral Salts come from earth’s crust by weathering – soluble ones enter into water cycle & eventually enter sea. Other ones returned to earth’s surface by sedimentation.
- Animals fulfil mineral requirement from mineral solutions or other animals.
- These are returned to soil after decomposed.
Biochemical cycles, also called biogeochemical cycles, describe the movement of chemical substances through the biological (biosphere), geological (lithosphere), and atmospheric systems of Earth. Understanding these cycles is crucial for UPSC aspirants as they underpin concepts of ecology, environment, and sustainable development.
The major biochemical cycles covered under the UPSC syllabus include the Carbon Cycle, Nitrogen Cycle, Phosphorus Cycle, Sulphur Cycle, and Water Cycle. These cycles help aspirants grasp how elements circulate and impact environmental processes.
The Nitrogen Cycle regulates the availability of nitrogen, an essential nutrient for plant growth. In India, disruptions in the nitrogen cycle due to excessive fertilizer use cause soil degradation and water pollution, affecting agriculture productivity and ecosystem balance—frequently discussed in UPSC exams.
The Carbon Cycle involves the exchange of carbon between the atmosphere, biosphere, oceans, and geosphere. It’s directly linked to climate change as increased atmospheric carbon dioxide from human activities contributes to global warming. Grasping this cycle aids aspirants in addressing climate-related policies and questions in UPSC exams.
The Phosphorus Cycle is unique because it doesn’t include a gaseous phase, and phosphorus typically cycles through land, water, and organisms at a slower rate. Understanding this helps UPSC aspirants address questions on nutrient management, soil fertility, and ecosystem dynamics.